Top Benefits of Implementing QMS Software in Plastic Production

Plastic is a ubiquitous element in almost every walk of our existence. Right from toys to electronics, study, defence, medical, aerospace, research everywhere plastic plays an important role. Statistics present an astonishing figure about plastic industry.
According to market insights by Share.Market the global market size of plastic industry was USD 72 billion in 2023, and is expected to reach USD 1,050 billion by the end of 2033. That means it is growing at the rate of 4% CAGR in the given time frame.
Usage wise the global plastic usage is projected to reach by 1.2 billion metric ton by 2060.
An industry that provides employment to around 5 million employees worldwide, and move trillions globally face significant challenges. In the subsequent section we will discuss those challenges and find their solution.
Challenges Plastic Industry Face:
Product quality consistency –
Variations in raw materials, manufacturing processes, and finished products can lead to inconsistent quality. Plastic manufacturers must take necessary measures to deliver consistent quality to the consumers.
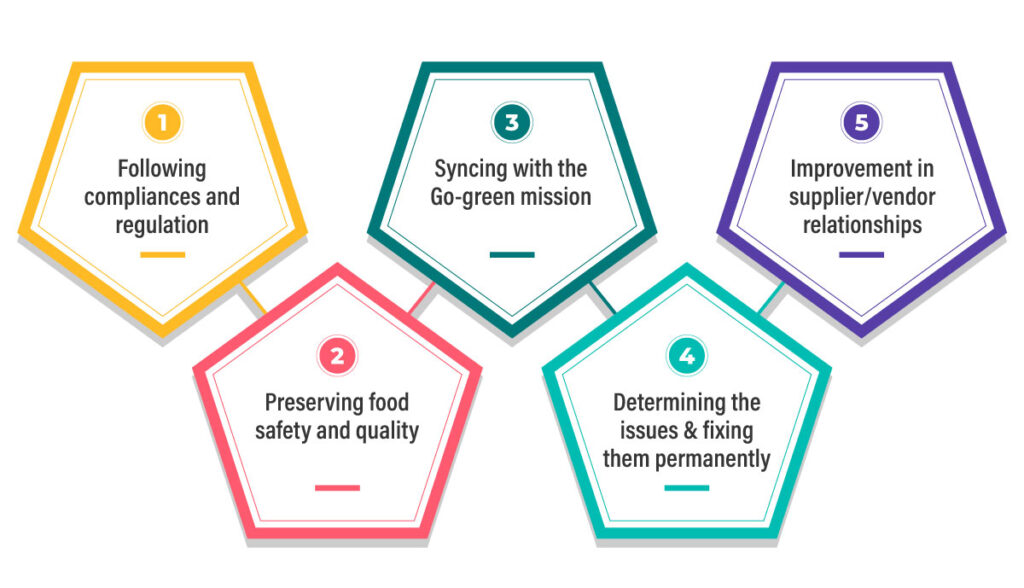
Regulatory compliance –
The plastic industry must meet stringent environmental and safety standards.
Waste management & sustainability –
Increasing environmental concerns across the world have heightened sustainability and waste management concerns of manufacturers. High plastic waste and inefficient resource use are major concerns for the industry.
Production downtime & defects –
Machine malfunctions and process deviations lead to costly downtime and rework. Many a times faulty calibration also lead to defects in the products.
Traceability & recall management –
In case of defective batches, tracking and recalling products efficiently is critical.
Documentation & change management –
Managing a high volume of technical documents and process changes can be complex. A slight mistake can lead to process halts, regulatory repercussions, or defective production.
Customer satisfaction & complaint handling –
Addressing and resolving customer complaints quickly is essential. Delayed resolution can fetch loss in brand reputation.
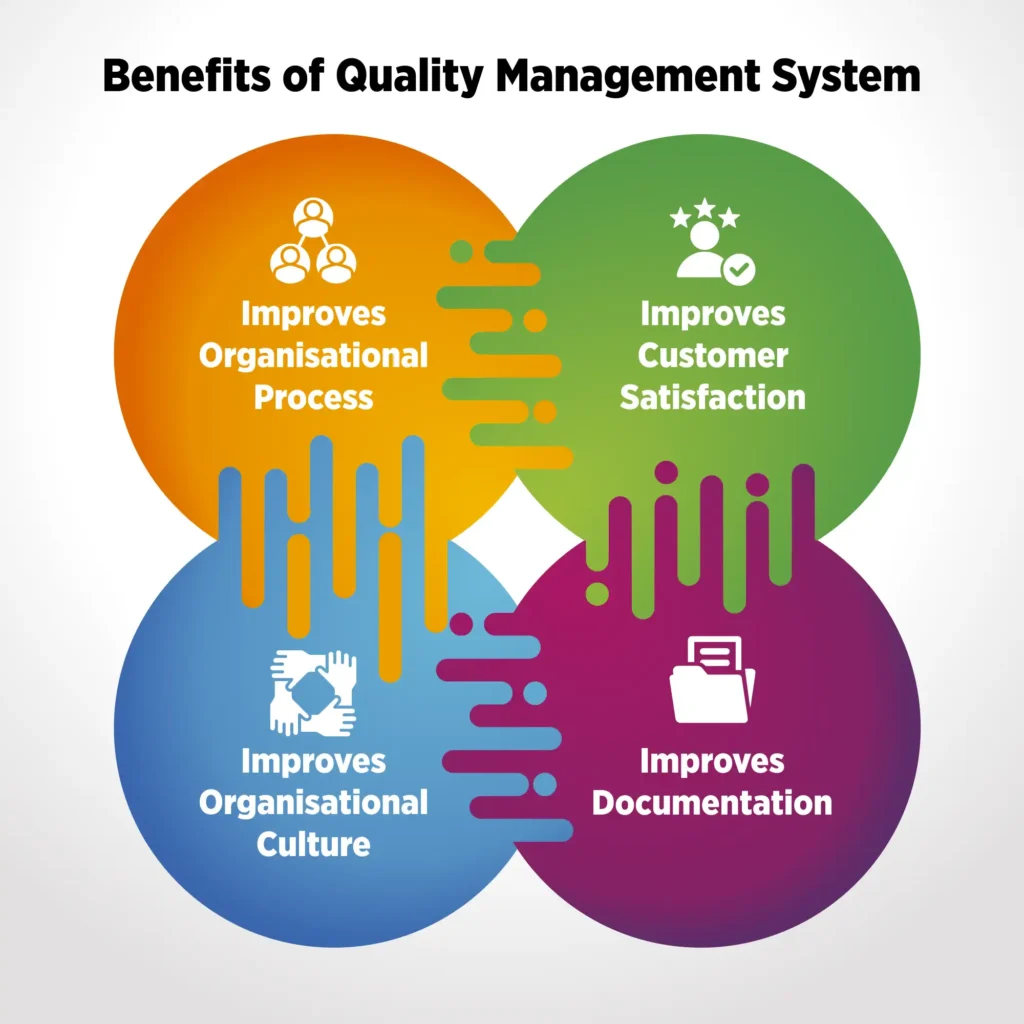
How Does QMS Software Benefit Plastic Production?
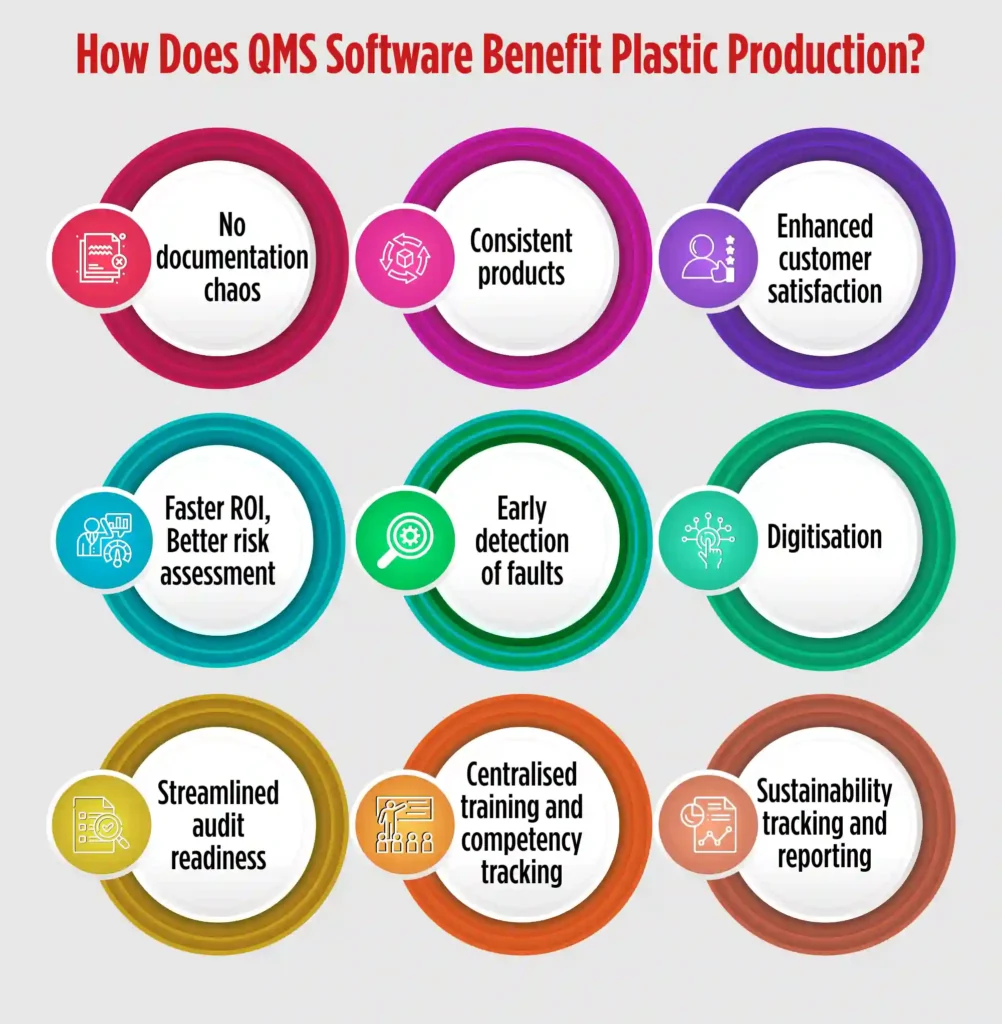
A plastic processing quality management software offers multiple benefits in plastic production. The software is designed to cater nuances of the quality management processes of the industry. There are multiple benefits plastic manufacturers reap with an industry oriented QMS software, such as:
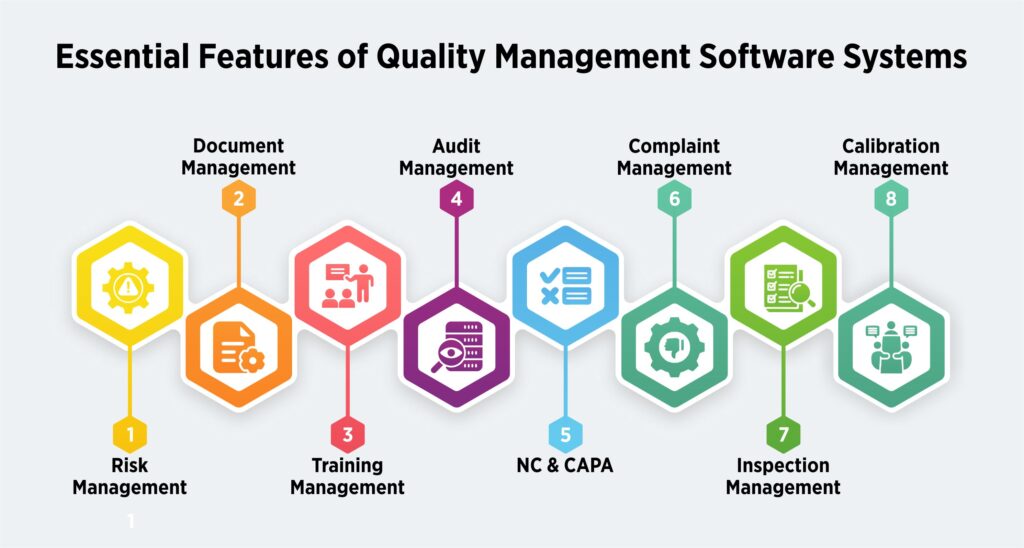
No documentation chaos– Quality solutions for plastic and packaging are designed to store digital version of all the documents related to plastic manufacturing, and tag and classify them for faster retrieval. So, regulatory documents, SOPs, Moulding/extrusion process parameter logs, employee training matrix, dispatch and packaging records, NOCs, details of pollution control devices, trade licences, etc. can be stored digitally and retrieved on simple clicks. Document Management System of QMS solutions, like QualityPro, can store as many documents as required, version them, and purge obsolete documents to avoid confusions.
Consistent products– QMS software standardises the processes of an organisation. It also puts a check on quality of materials used in the production process. Hence, by setting up a benchmark of processes and materials, QMS software ensures that all the products undergo the same processes and quality checks before reaching the customers. This strict check maintains consistency in products.
Enhanced customer satisfaction- As mentioned above, quality management software in plastic processing enhances consistency of products. The software also ensures that the complaints, if any, are resolved by the organisation in the best possible ways. A QMS software like QualityPro has a dedicated complaint management module that handles the complete complaint cycle — from raising a complaint to delegation and resolution. The module not only helps to make customers feel heard, but it also raises their satisfaction level.
Faster ROI- A QMS software for plastic production, such as QualityPro, is equipped with modules like Inspection Module and Calibration Module. Both the modules work in unison to enable manufacturers calibrate the equipment precisely and apply inspection checks on various raw materials, processes and products.
This ensures that there are fewer faults in the products which shortens the product to market cycle, eventually churning faster ROI.
Better risk assessment– Risk is an integral part of plastic production. Various risks hover the plastic production, like use of incorrect raw materials, inconsistent moulding parameters, uncalibrated machines, human error risks like missed inspection and so on.
A QMS for plastic production helps to assess, document, prepare, and strategies for risks. The Risk Management module in the QMS software helps to document risk, evaluate and categorise it based on severity. The module also helps to create an effective action plan and execute to mitigate risk.
Early detection of fault- Deviations from standard can arise due to many reasons and even an iota of deviation can cause product deformity or anomaly. What is important in plastic manufacturing is to detect the anomaly or deformity before it hits the market and take necessary action to avoid recurrence.
These deviations, called NCs, can be product-related like, flash or burrs one edges, discolouration or contamination, bubbles, voids or sink marks etc, or can be process-related like, improper cycle time, poorly mixed resins or additives, incorrect temperature or pressure settings in injection moulding etc.
A QMS solution for plastic production helps in NCs detection, application of corrective measures, and to carrying out preventive actions to avoid recurrence.
NC/CAPA module of the software empowers organisations to carry out NC/CAPA and deter faulty products enter the market.
Digitisation– Quality concerns become intense for organisations relying upon paper-based or spreadsheet- based traditional system. The QMS software, such as QualityPro, digitises entire processes, reducing processing time. Automation that comes handy with this digitisation minimises errors and boosts efficiency.
Streamlined audit readiness – In plastic production, regular audits are vital to ensure compliance with various regulatory bodies. A QMS software like QualityPro helps manufacturers stay audit-ready at all times.
From storing audit trails to tracking corrective and preventive actions post-audit, the software ensures that everything is documented, time-stamped, and easily accessible. This reduces stress during audit cycles and helps organisations clear audits without hassle.
Centralised training and competency tracking – Plastic manufacturing involves various complex machines and procedures. Ensuring that the workforce is trained and competent is crucial. QMS software like QualityPro includes a Training Management module that not only schedules training sessions but also maps employee competencies, assigns training based on role-specific needs, and tracks certifications. This ensures that only qualified personnel handle critical processes, reducing errors due to human factors.
Sustainability tracking and reporting – As the world focuses on reducing plastic waste, manufacturers are under pressure to adopt sustainable practices. QMS software enables tracking of sustainability KPIs such as scrap generation, resource consumption, recycling rates, and emission data.
With these metrics readily available, companies can generate sustainability reports, set improvement goals, and demonstrate their commitment to greener practices to clients and regulators alike.
Concluding Thought:
In an era where precision, compliance, and sustainability define business success, plastic manufacturers cannot afford to rely on outdated quality practices. Implementing a robust QMS software not only addresses the industry’s critical challenges but also empowers manufacturers to lead with consistency, agility, and confidence.
From reducing defects and downtime to strengthening traceability and regulatory compliance, a purpose-built QMS transforms quality from a reactive task to a proactive advantage.
With QualityPro QMS software, plastic manufacturers gain a strategic partner that streamlines operations, ensures audit readiness, and drives continuous improvement—ultimately enhancing product quality, customer trust, and business growth.
If you are a plastic manufacturer looking forward to upgrade your quality game, QualityPro is the best investment you can make. To know more and to book a free demo of the product, visit QualityPro.