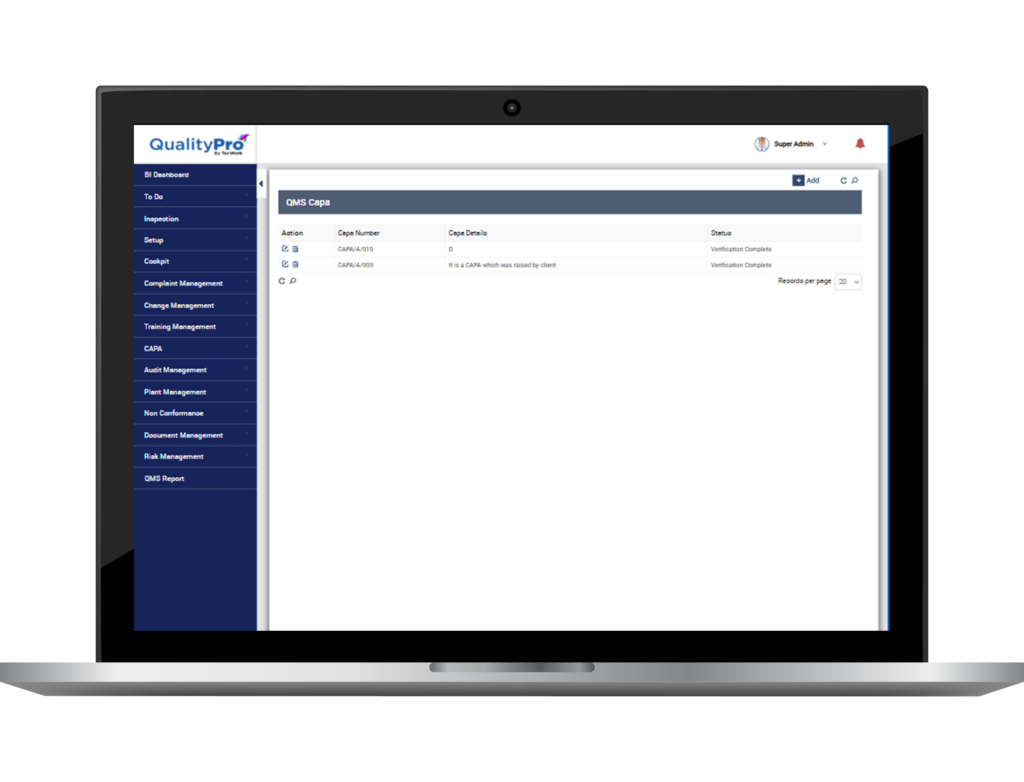
Nonconformance (NC) and CAPA Management System
Instil a Culture of Continuous Improvement
Analyse and Avoid Issues
When a product or service does not meet the set standard of quality, it is said to be nonconformance (NC). A Nonconformance and CAPA management software is a tool which identifies, documents, analyses and segregates NC for further investigation and follow ups.
Correction and Preventive Action (CAPA) is a concept which systematically mitigates anomalies, or inconsistencies and ensures steps to prevent its recurrence. It is a well elaborated system of process used to ensure conformity with quality standards and regulatory laws.
QualityPro is a robust eQMS software, offering an efficient NC and CAPA module. The comprehensive module helps to track anomalies, document them, attach proofs and relevant documents, find root cause, rectify the errors, and instil continuous improvement in the organisation.
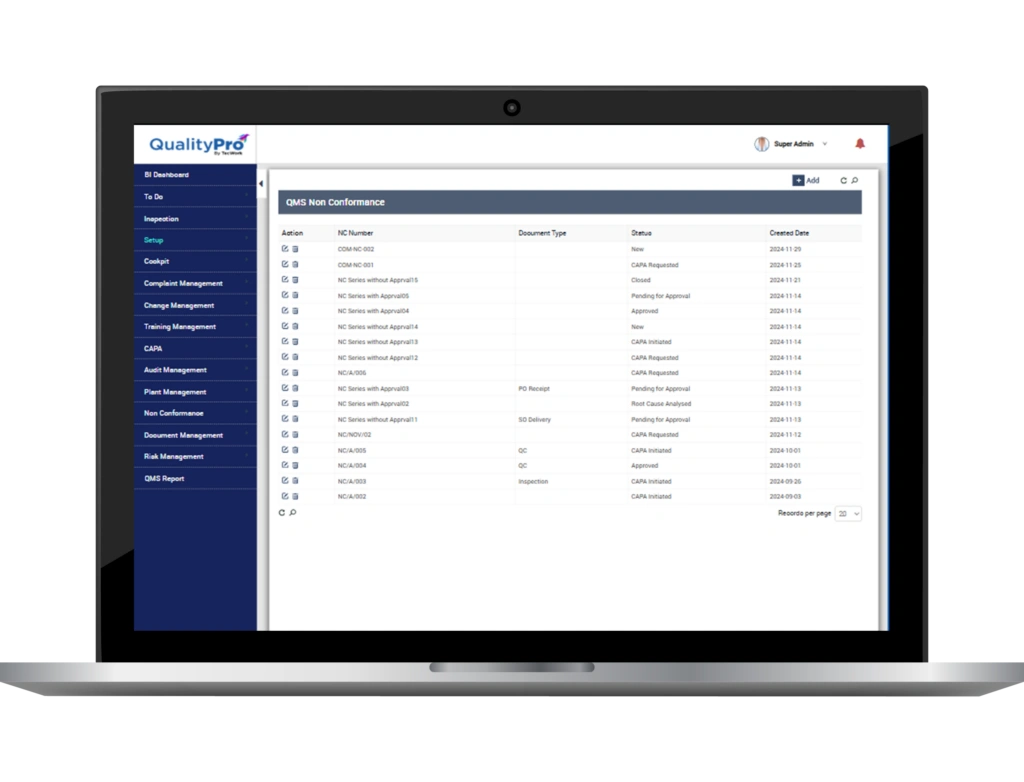

Manage Nonconformance (NC) and CAPA Process with Ease
- Collect data from complaints, nonconformances, anomalies raised due to non-conformances, deviations, incidents, internal/external audits, complaints and more
- Log an issue, upload evidence, classify type, associate severity, and assign it to the concerned authority
- Maintain and track nonconformity records
- Establish a regulatory-compliant Corrective and Preventive Action (CAPA) workflow procedure, complete with defined action routes
- Streamline and automate CAPA implementation by digitising CAPA related documents, forms and approvals
- Assign tasks and responsibilities to team and individuals to carry out CAPA
Your path to better quality management starts here
Gain Insight
- Get snapshot of customer relationship and visually analyse customer complaints with dashboard.
- Get details of NC, complaints or anomalies and track their frequency.
- Have on-click important data like- open CAPAs, pattern of anomalies, pending tasks and approvals and more.
- Trace potential quality issue from start to end with uniform dissemination of facts.
- Connect easily with other modules like SOP, Complaint Management, Audit Management, Change Management etc for speedier actions.
- Identify the areas to be impacted or affected with CAPA and pre-inform the concerned departments for faster actions.
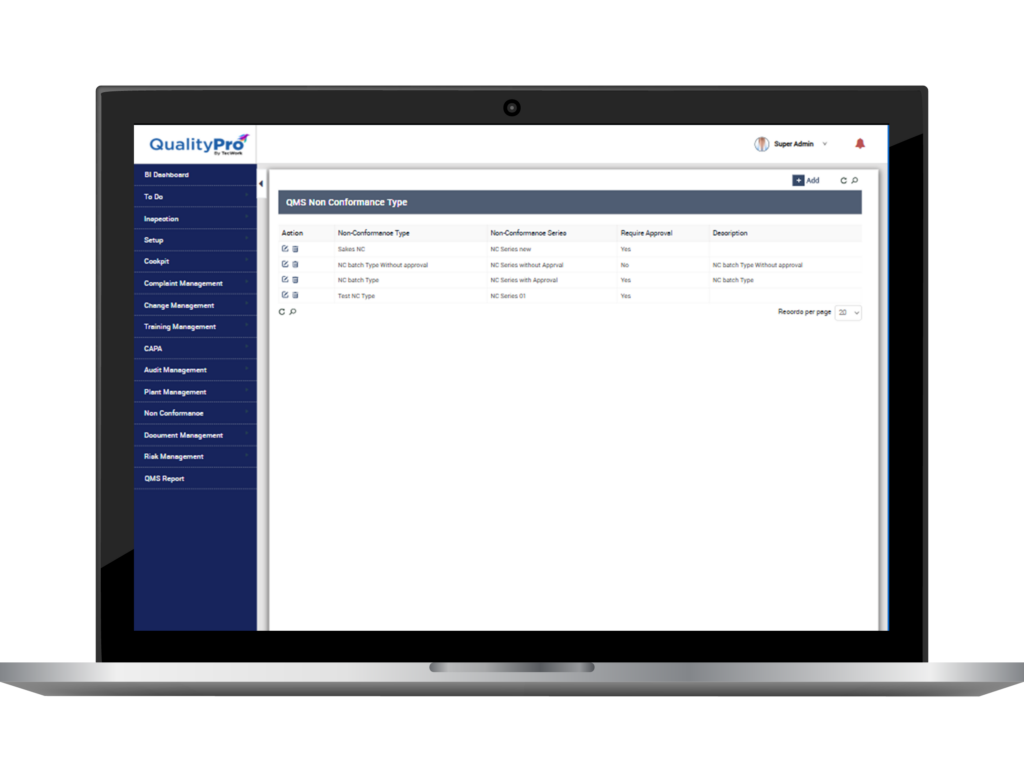
Don’t just take our word for it—Experience QualityPro firsthand
Gain Insight
- Get snapshot of customer relationship and visually analyse customer complaints with dashboard.
- Get details of NC, complaints or anomalies and track their frequency.
- Have on-click important data like- open CAPAs, pattern of anomalies, pending tasks and approvals and more.
- Trace potential quality issue from start to end with uniform dissemination of facts.
- Connect easily with other modules like SOP, Complaint Management, Audit Management, Change Management etc for speedier actions.
- Identify the areas to be impacted or affected with CAPA and pre-inform the concerned departments for faster actions.

Find, Rectify, Improve
- Identify the issues, document it, collect data and investigate to find the precise trigger of Nonconformance (NC).
- Send alerts to all impacted departments and achieve continuous improvement by refining processes, enhancing training, strengthening quality control measures, etc.
- Link it with other areas like training, document management, change management for rectification.
- Link current CAPA instances with existing CAPA instances for quick actions and remedy.
- Attach supporting documents in a snap.
Benefits of Nonconformance (NC) and CAPA Module
- Reduces quality costs
- Assures consistency
- Enhances quality assurance
- Improves risk response
- Stronger compliance
- Reduces product recalls
- Eliminates scrap
- Reduces unfortunate events
- Reduces reworks and repair costs
- Reduces returns and restocking costs
- Minimises warranty expenses