
- Overview
- What is Electronic Quality Management System?
- Difference between QMS and eQMS
- Develop a Culture of Quality
- What are the Features of an Electronic Quality Management System?
- What are the Benefits of Electronic Quality Management Software?
- Final Thoughts
Overview
Historically, paper-based and spreadsheet-centric quality systems were used to monitor and analyze quality-related data. Manual data entries and disconnected files made it difficult to track and manage quality-related information. Regarding challenges, while addressing top-quality management objectives for life sciences industry, respondent results were:
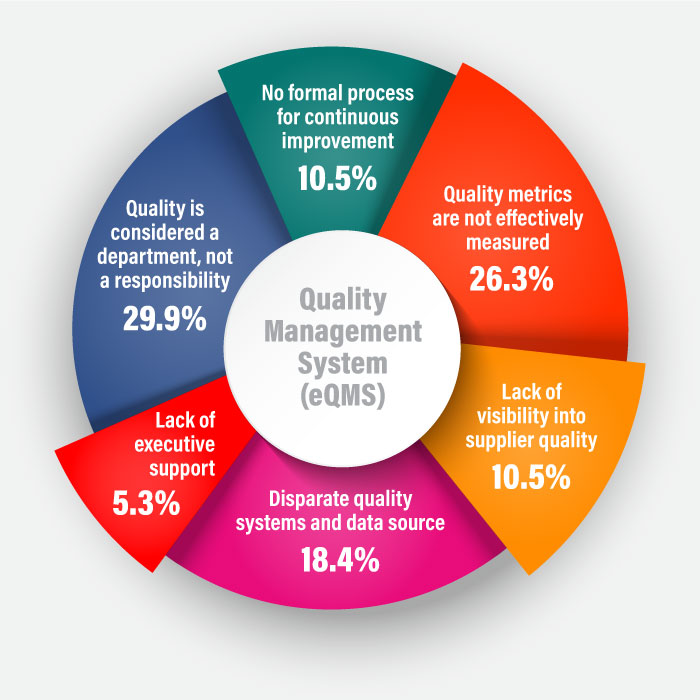
All thanks to the evolution of integrated IT-based systems like eQMS that made it easier to record and report quality variables across various access points. This blog discusses the definition, functions, benefits, and future of eQMS software with respect to total quality management.
What is Electronic Quality Management System?
An Electronic Quality Management System (eQMS) is a software solution designed to electronically handle, document, and manage quality-related processes within an organization. Its primary objective is to ensure that products meet required standards, are free from defects, and operate within defined tolerances.
This software encompasses a range of techniques, structures, processes, and resources necessary for efficient Total Quality Management (TQM) practices. By leveraging an eQMS, organizations aim to enhance process efficiency and minimize human error.
One of its key advantages lies in its ability to proactively identify potential quality issues before they manifest. It grants enhanced visibility into Quality Assurance (QA) and Quality Control (QC) processes within a manufacturing organization.
Core functionalities of an eQMS include compliance management, change management, investigation of adverse events, initiating corrective and preventive actions, managing risks, and integrating complaint management into the quality process.
The system operates as a closed-loop, ensuring accountability at every level of the quality management process.
eQMS software can be implemented through various deployment models such as on-premise, cloud-based, or hybrid setups. This flexibility allows organizations to choose based on their data access requirements.
Furthermore, these systems often offer integration capabilities with other business management software such as Enterprise Resource Planning (ERP), Customer Relationship Management (CRM), Product Lifecycle Management (PLM), and Manufacturing Execution Systems (MES) to streamline operations across different departments and processes within the organization.
Difference between QMS and eQMS
The difference between a Quality Management System (QMS) and an Electronic Quality Management System (eQMS) lies primarily in their operational modes, capabilities, and efficiency in managing quality-related processes:
Operational Approach:
- QMS: Traditionally, QMS may rely on manual processes, spreadsheets, or paper-based documentation to manage quality-related operations. These methods can work independently, potentially leading to discrepancies and various versions of information.
- eQMS: eQMS integrates all quality management functions into a single, unified software system. It stores and manages all quality-related data in a centralized location, offering a single source of truth and reducing the risk of version discrepancies.
Data Accessibility and Security:
- QMS: Paper-based or spreadsheet-centric QMS can limit access to updated documents and lack a comprehensive record of changes.
- eQMS: Ensures access to updated, authorized documents, and maintains an audit trail of all changes. Unauthorized or unsigned documents are flagged, enhancing data security and compliance.
Regulatory Compliance:
- QMS: Managing regulatory compliance can be challenging with traditional QMS, potentially leading to compliance issues due to manual processes and inadequate documentation.
- eQMS: Provides convenience and ease in handling regulatory compliance documentation by offering features specifically designed to manage compliance requirements effectively.
Develop a Culture of Quality…
“55% of respondents who don’t own enterprise quality management software (eQMS) say their quality metrics aren’t properly measured.”- LNS Report
The effective utilization of an Enterprise Quality Management Software (eQMS) is pivotal in fostering a pervasive culture of quality within an organization. When every individual within the company embraces a quality-centric approach and resources are aligned to deliver superior products, it reinforces a culture committed to excellence.
Specifically designed to optimize various quality-related aspects in manufacturing, eQMS software ensures streamlined maintenance of factory equipment, meticulous inspection of raw materials and finished goods, and proficient handling of quality audits.
Successful eQMS implementation yields numerous benefits, including heightened product quality, reduced product recalls, minimized wastage, and fewer warranty claims, all contributing to improved operational efficiency.
eQMS systems offer robust data integration capabilities, consolidating quality-related information into a unified database. This integration drives continual improvement across the entire product life cycle.
Additionally, eQMS solutions are conveniently scalable, adapting seamlessly to the growing needs of the manufacturing organization. By eliminating departmental silos and establishing automated alerts for immediate attention to quality issues, eQMS promotes accountability.
Addressing the culture of quality, ISO 9001:2015 serves as the primary regulatory guideline, ensuring the adherence to specified quality standards across the organization.
eQMS software aligns with ISO 9001:2015 requirements, ensuring that processes, individuals, systems, resources, and final products meet the stipulated standards. This guideline outlines key characteristics for a company’s culture, emphasizing quality in business processes, workforce efforts, and the end product.
What are the Features of an Electronic Quality Management System?
Specific facets of total quality management require specific features to be included in the eQMS system. Below listed are the most important functions of eQMS:
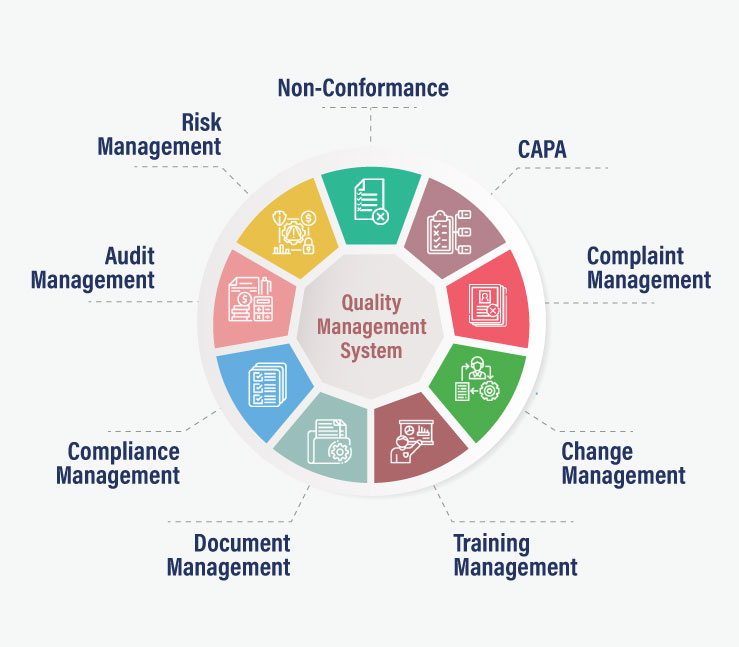
- Non-Conformance – It is important to identify product issues by testing for quality and eQMS helps the organization identify events of non-conformance in following the SOPs. Valid issues are considered for further actions, while invalid issues are terminated on the spot. eQMS also records all the actions taken to correct the non-conformity.
- CAPA – When a non-conformance issue is identified and found to be valid, eQMS initiates the process of improvement. It specifies the corrective actions that need to be taken in terms of change in process and the preventive action required to rework/scrap the product, so that the final product quality is maintained up to the standards.
- Complaint Management – The eQMS software helps manufacturing organizations establish a complaint handling process. The entire complaint management life cycle from documenting the problem, performing root cause analysis, providing timely response to the customer, and triggering internal CAPA is integrated into the eQMS system.
- Change Management – When a process change is required or a CAPA issue is raised, the eQMS system manages and documents the entire change control process. It helps businesses automate the entire change management process starting from initiating change requests, impact evaluation, actual implementation, and culminating with verification and closure.
- Training Management – eQMS helps organizations keep their employees updated with the latest certifications on all controlled documents in the organization. It helps manager identify training needs and keeps track of employee performance throughout the process. The eQMS software can also issue certificates on completion of the training modules.
- Document Management – eQMS manages all the critical documents of the organization from creation to approval & publishing to archival. Along with updating to the latest version of the documents, eQMS also facilitates the removal of obsolete files. It can also create a master list and document map to track document changes.
- Compliance Management – eQMS can help manufacturers by maintaining a clear list of compliance requirements. It automates the tracking and control of compliance-related activities like documentation, planning, reporting, audit, etc. eQMS also simplifies the reporting of non-compliance events to save the company from legislative action.
- Risk Management – eQMS takes a very structured approach to identify, assess, control, communicate and review all the quality risks in the organization. With eQMS, organizations can take actions to ensure that any anticipated problems don’t occur and if they do, have a control mechanism to rectify them.
- Audit Management – eQMS helps organizations conduct internal and external audits to enhance product quality. It can schedule, structure, perform and assess periodic and ad-hoc audits within the specified time frame. The audit management functionality also helps organizations meet industry-specific compliance requirements.
What are the Benefits of Electronic Quality Management Software?
An eQMS can transform the manufacturing organization in terms of men, machines, materials and methods. Below are the major benefits of an eQMS:
- Enhanced Effectiveness: Clearly defining and achieving quality objectives set by the organization.
- Cultivating a Quality Culture: Instilling a values-based system focused on maintaining high-quality standards across the organization.
- Streamlined Data Management: Establishing a centralized database for consistent and accurate data management, ensuring a single source of truth.
- Enhanced Accessibility: Enabling access to the system from multiple devices such as laptops, smartphones, and tablets.
- Improved Audit Functionality: Setting tolerance levels and creating audit trails to facilitate effective audit processes.
- Increased Customer Satisfaction: Adhering to quality policies and implementing streamlined processes directly impacts customer satisfaction.
- Enhanced Compliance: Maintaining updated compliance records and automating alerts to ensure adherence to regulations.
- Improved Documentation: Defining and documenting critical processes and objectives systematically for better clarity and understanding.
Final thoughts
eQMS focuses on the consistency of the process. It ensures that every time a production cycle is performed, the same information, material, methods, skills & controls are applied in a constant manner. Along with heightened productivity, an electronic qms software helps prevent costly mistakes, enhance product management, improve risk forecasting and establish quality metrics. Therefore, it is important for organizations to implement an eQMS and QualityMaster can prove to be a perfect choice.
Get QualityMaster today and enjoy the benefits of end-to-end quality management. Feel free to write to us at contact@bmqualitymaster.com, or contact us to know more about it as well as our other products.


