In the fast-paced world of business today, document management is vital for productivity and compliance. Document version control, also known as versioning, plays a pivotal role in this process. It enables the tracking and management of document changes over time, ensuring accuracy, compliance, and seamless collaboration among team members.
Yet, managing versions manually can be cumbersome and prone to human errors, leading to version chaos. Document Management Software (DMS) makes things easier by putting all documents in one place, controlling versions, managing edits, and keeping track of changes. With DMS software, keeping documents up-to-date and compliant has never been easier.
Result: Smoother and reliable document management with 100% accuracy.
If you’re eager to explore version control, its best practices, and how DMS software simplifies it, then keep reading the blog. Something interesting is waiting for you at the end!
Table of Contents:
- What is Version Control?
- What do we need for Document Version Control?
- Interesting Facts & Figures
- Best Practices for Document Version Control
- The Challenges of Managing Document Versions in a Manufacturing Business
- What is Document Management Software and how does it help with Version control?
- How QualityMaster Enhances Version Control?
What is Version Control?
Version control tracks different document versions and changes made over time. Imagine you are working with a team on a project. You are constantly updating and refining a document. This document could be a company policy or a research paper.
Version control tracks every change in a document, from the first draft to the final polished version. It records addition, deletion or modification, creating a sort of timeline or history. This is incredibly valuable, especially in collaborative efforts, because it lets you know who did what and when they did it.
Whether it’s correcting a typo, adding new information, or suggesting improvements, it ensures transparency in the entire document’s evolution, making it simple to review past decisions and understand why certain changes were made.
Version control is similar to a document’s diary. It records the document’s growth and assists in managing it effectively. Multiple individuals find this particularly useful when they are involved in its development.
What do we need for Document Version Control?
To successfully implement document version control, you should have the following elements in place:
- Clear Versioning System: You’ll need a standardized way to label different versions of your documents. For instance, you can use numbers (like 1.0, 1.1, 2.0), letters (A, B, C), or a combination of both.
- Audit Trail: A system that keeps track of and records all changes made to a document. This includes the person who made the changes, the time when they made them, and the specific changes that were made.
- Access Control: Permissions and controls to limit who can edit a document, ensuring only authorized individuals can make modifications.
- Storage and Retrieval: A secure place or document management system to safely store, organize, and easily find documents.
- Collaboration Tools: Tools and features that make it easy for teams to work together on documents while still maintaining version control. For example, a check-in/check-out system can help prevent conflicts when multiple people are editing the same document.
Interesting Facts & Figures-
To illustrate the importance of document version control, consider these statistics:
- According to a McKinsey report, employees spend 1.8 hours every day searching and gathering information. On average, that’s 9.3 hours per week!
- In regulated industries like healthcare and pharmaceuticals, document version control is critical for compliance. Failure to comply with regulations can result in hefty fines.
For example, in 2020, the U.S. Department of Health and Human Services issued over $36 million in fines related to HIPAA violations.
Best Practices for Document Version Control-
Proper version control helps organizations maintain consistency, track changes, and mitigate errors. Here are some key reasons why implementing best practices for document version control is crucial:
Appoint an Administrator: Hire an EDMS administrator to oversee document management, compliance, and system maintenance.
Maintain Document History: Keep a clear version history to track changes and easily revert to previous versions, when needed.
Secure Documents: Use “check-out” features to protect documents from unauthorized edits and unnecessary revisions.
Member Controls: Implement precise access controls to restrict editing based on team roles.
Workflow Automation: Set up standardized workflows for tasks like watermarking, PDF generation, and electronic signatures.
Device Accessibility: Choose an accessible eDMS that’s interoperable from on computers, tablets, as well as smartphones.
Enhance Security: Implement encryption and backup plans to ensure document security and data integrity.
Integration: Connect your version control system with existing IT systems for better document management.
The Challenges of Managing Document Versions in a Manufacturing Business
The challenges that you might face while managing document versions manually are as follows:
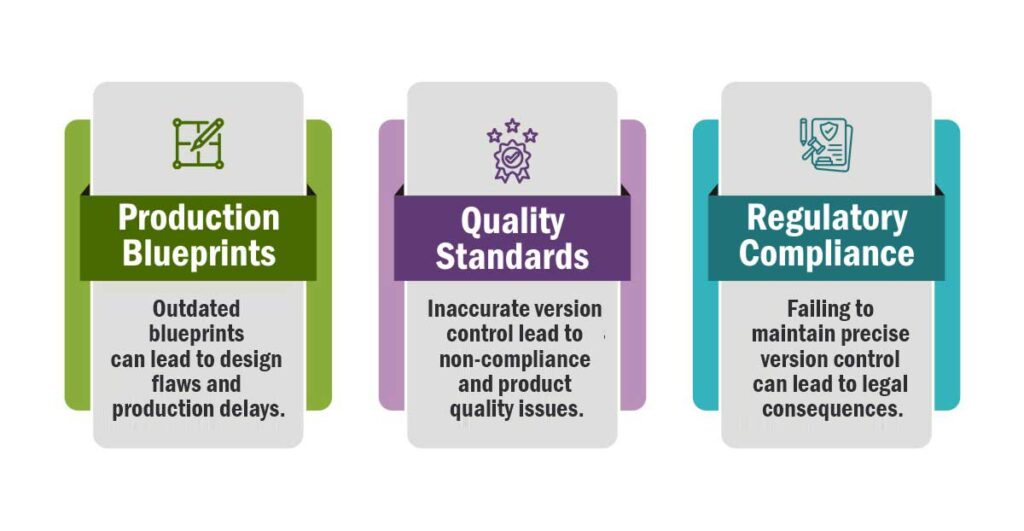
Production Blueprints:
In manufacturing, detailed blueprints and engineering drawings are essential. When manual version control is in place, it’s easy for errors to occur. For instance, using an outdated blueprint in the production process might lead to a costly error in the final product.
Example:
Imagine a car manufacturer working on a new model. If the team doesn’t use automated version control, they might use an old blueprint by mistake. This can cause design problems and delays in production.
Quality Standards:
If multiple versions of a quality control manual exist, it can be challenging to ensure that workers are following the correct procedures, potentially leading to subpar products or safety issues.
Example:
Consider a food processing plant. Having multiple versions of the quality control guidelines can lead to workers being unaware of the latest safety procedures. This lack of knowledge increases the risk of contamination or product recalls.
Regulatory Compliance:
Manufacturing businesses often operate under strict regulatory frameworks, such as ISO certifications or environmental standards. Failing to maintain precise version control can result in non-compliance and legal consequences.
Example:
In the aerospace industry, keeping track of the latest versions of documents related to safety and environmental regulations is crucial. Outdated versions could result in fines or even the suspension of operations.
Therefore, manufacturing companies often turn to automated Document Management Software to ensure precision, compliance, and efficiency in their operations.
What is Document Management Software and how does it help with Version control?
A Document Management System (DMS) is a digital tool that helps store, organize, and track documents. It ensures easy access, security, and version control. Document version control is crucial for managing information effectively within organizations.
It ensures that everyone works with the most current and accurate documents. Here’s how a Document Management System (DMS) enhances this process:
- Accuracy: A DMS software maintains a single, updated version of each document, eliminating confusion caused by multiple versions circulating within a team.
- Compliance: It tracks changes and provides an audit trail, simplifying compliance with industry regulations by ensuring documents adhere to necessary standards.
- Efficiency: DMS software enables real-time collaborative work, allowing multiple users to edit documents simultaneously. It also preserves version history for easy tracking of changes.
- Confidence: Employees can trust the documents they access, knowing they are the latest, approved versions. This confidence boosts productivity and decision-making.
Document Management Software automates these processes, ensuring documents are secure, easily accessible, and always up to date. One such automated document management system is QualityMaster.
How QualityMaster Enhances Version Control?
QualityMaster is a robust electronic Quality Management System (eQMS) that significantly enhances version control of documents and processes within an organization. Here’s how QualityMaster achieves this:
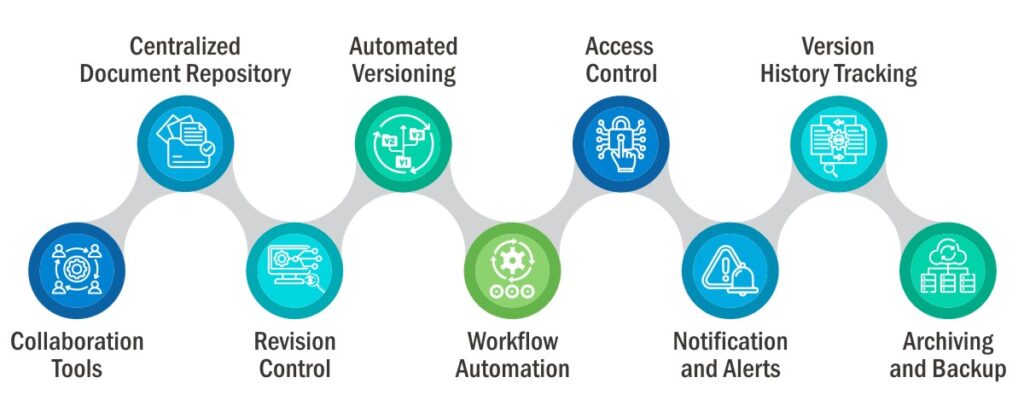
- Centralized Document Repository: QualityMaster provides a centralized repository for all documents, including policies, procedures, and work instructions. This ensures that all users access documents from a single, secure location, minimizing the risk of multiple versions floating around.
- Automated Versioning: The system automates version numbering or naming, eliminating manual errors in labeling documents. It assigns version numbers or names consistently, making it easy to differentiate between document versions.
- Access Control: QualityMaster allows administrators to define access permissions for users and groups. This means that only authorized employees can edit documents, ensuring version integrity and document security.
- Version History Tracking: The system maintains a detailed version history for each document. It keeps a record of who made changes, when they made them, and the nature of the modifications. Users can easily review the document’s evolution and track all changes over time.
- Collaboration Tools: QualityMaster facilitates collaboration among team members. Multiple users can work on the same document simultaneously, and the system seamlessly merges changes. This ensures that all contributions are incorporated into the final document.
- Revision Control: The system enforces strict revision control, allowing organizations to manage document changes efficiently. Users can submit proposed changes for review and approval, ensuring that only authorized modifications are accepted.
- Workflow Automation: QualityMaster streamlines document approval processes through workflow automation. This ensures that documents move through the approval chain smoothly and that the right individuals review and authorize changes.
- Notification and Alerts: QualityMaster can send notifications and alerts to users when document changes occur or when approvals are required. This keeps users informed and ensures timely responses to document modifications.
- Archiving and Backup: The system includes archiving and backup features, allowing organizations to retain historical document data. This ensures that previous versions are accessible if needed for reference or auditing purposes.
Concluding Thoughts,
So, are you ready to elevate your document version control? Take the next step toward enhanced accuracy, compliance, and efficiency with us. Contact today to learn more and start optimizing your document management process.
Feel free to write to us or visit our website to know more about it as well as our other products.


