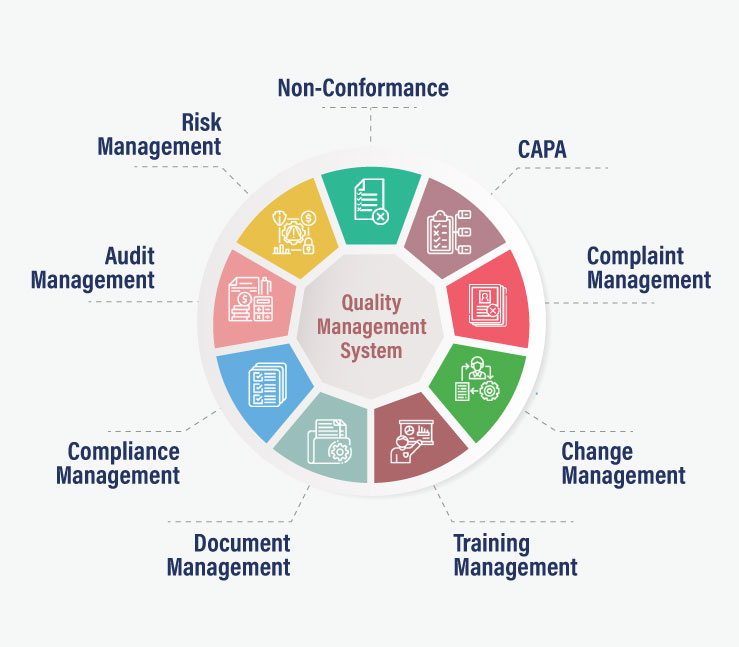
What is Non-Conformance Software?

What if a food processing plant finds that the packaging of one of its products was contaminated with bacteria during production? Or, the pharma company finds that the manufactured drug batches have the wrong dosage prescribed.
In situation like these, the possible consequences that might come across the manufacturers will be – re-work on the product, scrapping out the wrong ones, time & resource wastage and many a times wrong word of mouth for the brands which could also lead to unsatisfied customers and legal penalties.
Long story short, inability to determine non-conformance is the whole-sole reason manufacturers face situations like these. Keep on reading the blog to explore what it is and how manufacturers can prevent themselves from sliding into situations like these.
Table of Contents:
- What is Non-Conformance?
- What consequences does non-conformance have?
- Non-conformance management as per ISO 9001 standard
- Tips for mitigating non-conformance
- How to tackle Non-Conformance?
What is Non-Conformance?
Non-conformance is an indication that a service, process, product, or even the system has went wrong because it didn’t meet a specified set of requirements. A non-conformance indicates that a company’s standard operating procedures perhaps need to be modified or updated in some ways, or both. These deviations can be identified by conducting internal and external audits, analyzing customer complaints, material inspection or routine testing.
After that, a non-conformance report is created. A Non-Conformance Report provides structured documentation of the specifics of a Non-Conformance found. As a result, entities, initiatives, or individuals that fall short of the required standards in terms of quality and safety may be held liable.
This report’s objective is to record the specifics of a departure from expectations. This report assists management in providing a clear and logical definition of the issue so that appropriate steps can be taken to accomplish the desired improvements.
A non-conformance management software ensures that each non-conformance is accurately recorded and addressed with. It helps businesses in upholding quality standards and fostering growth. The module can function independently or in conjunction with our other quality control software.
What Are the Different Types of Non-Conformances?
Basically, there are two categories of non-conformance:
- Minor Non-Conformance
Minor non-conformance arises on a small level and doesn’t affect the whole company. Therefore, this kind of nonconformance has small repercussions and is much simpler to resolve. For example, small fixes in product manuals. This can easily be fixed by updating the relevant documentation.
A minor non-conformance is typically not a big deal and is easy to fix. Minor non-conformances could, however, grow bigger if they are neglected. Businesses should have systems and processes in place for recognizing and resolving non-conformances in order to prevent minor issues from developing into serious ones. Some more examples of non-conformance are:
- Multiple violations of requirements.
- Multiple missing/unsigned documents
- Improper machine calibration
- Major Non-Conformance
The entire production or workflow of the company is significantly impacted by a major non-conformance. The end product could be significantly degraded by serious non-conformance issues. Things like untested items or making unauthorized, unapproved changes to the production without documenting, monitoring and communicating the impact of changes are examples of serious non-conformance.
A major non-conformance can have a significant negative impact on a company’s or organization’s reputation and brand. These are more challenging, expensive, and time-consuming to fix than a minor non-conformance. Therefore, tools and processes that are effective in identifying and resolving serious non-conformances before they have a negative impact on their operations are essential.
What consequences does non-conformance have?
If non-conformances are not identified and handled on time, they can create chaos and havoc. For example, failing to address a change to all the concerned departments may lead to incorrect functioning of your employees and thus processes going haphazard. The morale of your organization can suffer as a result of persistent personnel actions that violate ISO 9001 criteria.
Additionally, manufacturing goods that don’t meet criteria can invite rework, scraping, even product recalls, legal suits and what all follows this is obvious. But these consequences can be possibly reduced with the help of a Non-Conformance Management Software.

Non-conformance management as per ISO 9001 standard
The management of these events can be connected to any of the current ISO standards, with ISO 9001 being one of the most popular. In order to constantly enhance operations, improve the quality of output, foster efficiency and competitiveness, and increase customer satisfaction, numerous industrial enterprises have embraced the globally recognized ISO 9001:2015 quality management system.
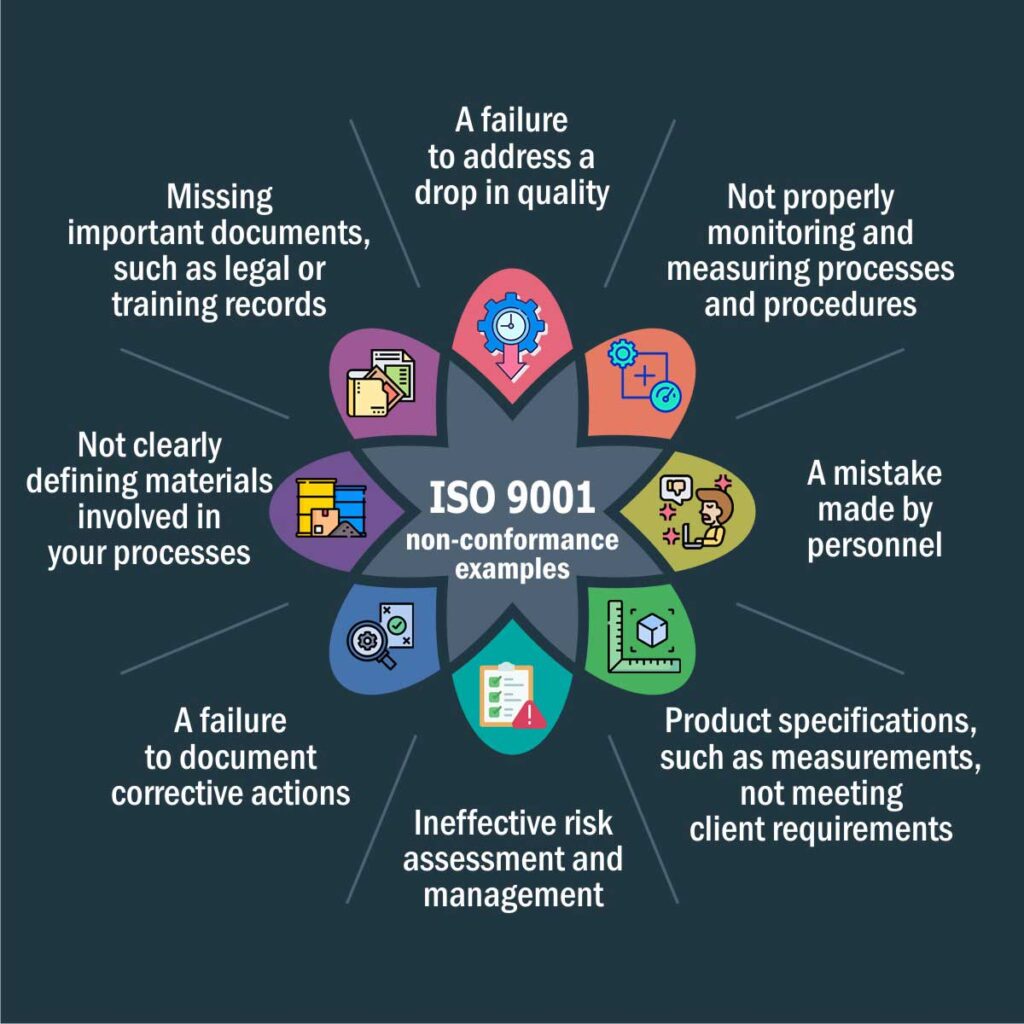
Failure to adhere to the technical specifications of the products (characteristics, materials, operation, etc.) or of the processes results in the majority of non-conformances within an ISO 9001 management system (delays in delivery times, errors in quantities, human failure due to lack of training, inadequate use of equipment, etc.).
Tips for mitigating non-conformance
It is crucial to set up suitable procedures and vital measures intended to stop Non-Conformance before it occurs. To do this, there are a number of actions to followed:
- Finding the non-conformance
A non-conformance can be found by any organization’s stakeholder, including customers, internal employees, suppliers, external auditors, and public administration. This must be disclosed as quickly as possible, whether it occurs via internal or external audits, quality controls, or analysis of consumer complaints or claims.
It is crucial to accurately describe the non-conformance during this initial stage of the process and to provide information, such as the proof that the non-conformance occurred, the document outlining what should have been done but wasn’t, the time and individual who discovered the non-conformance, etc.
- Resolution of the non-conformance
Once the non-conformance has been identified and thoroughly documented, it must be fixed in order to quickly resolve the issues and the reason that caused it. The work is not finished yet; as it might not be a one-time issue and is can spring-up often; thus, it will be important to address the core cause.
- Detection and investigation of the non-conformance’s root causes
In order to prevent future replication of the non-conformance, this is a crucial step. There are various effective approaches, such as the problem analysis and resolution tool, for identifying and treating the root cause (there may be one or more than one).
- Planning and implementing corrective measures to eliminate the issue
Once the underlying reason of the non-conformance is identified, businesses can take one or more corrective measures to mitigate it, take charge of implementing those measures and decide a timeline for doing so.
- Verification of the corrective action
The effectiveness of the corrective activities put in place will be determined in this final stage, which is one of the most crucial ones. Check that the deviation no longer occurs as soon as a circumstance identical to the one that caused the non-conformance surfaces.
Remember to solve the problem until you can confirm with absolute certainty that the remedial measure is working.
How to tackle Non-Conformance?
Implementing a Non-Conformance Software or a QMS system integrated with NC module can help you track non-conformances, identify the core issues and speed up the process of implementing the corrective actions in the business operations.
With QualityMaster NC/CAPA modules, businesses can obtain real-time data to detect non-conformances at the earliest and minimize their potential impact. In addition, they can have detailed information and tools for root cause analysis of the non-conformance and take corrective and preventive solution to eradicate it and never let the issue occur again.
With this solution, companies increase the agility with which they make decisions and execute corrective actions; experience improved competitiveness and customer service levels; reduce non-quality costs, and comply with the company’s continuous improvement strategy.
Want to make Non-Conformance Management easier, faster, and more structured?
Deploy QualityMaster now!! For more details, visit https://www.bmqualitymaster.com/contact-us/ or mail at: contact@bmqualitymaster.com